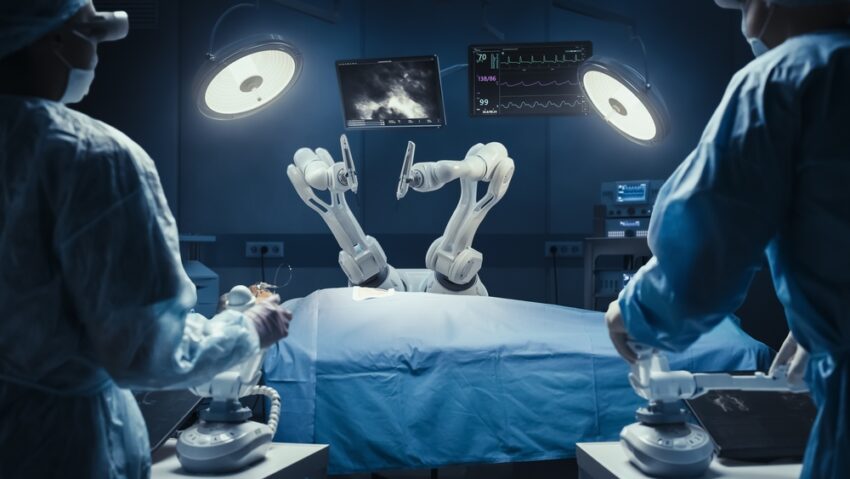
CMR Surgical, a Cambridge-based medtech company, has achieved a major breakthrough after receiving marketing authorisation from the US Food and Drug Administration (FDA) for its portable Versius Surgical System.
This marks a significant step into the world’s largest healthcare market, enabling CMR to prepare for sales of the Versius system in the US, initially for gallbladder removal surgeries in adult patients aged 22 and above.
The Versius system, designed to replicate the movements of the human arm and enhance surgeon precision, is already the second-most widely used robotic surgical system globally, with more than 26,000 surgeries completed, including in the UK. This approval comes nearly a decade after CMR Surgical was founded in 2014.
CMR Surgical’s headquarters and manufacturing site remain in Cambridge, with backing from international investors, including the Japanese tech giant SoftBank and China’s Tencent. The company, which has raised approximately $1 billion since its inception, employs over 500 staff, 400 of whom are based in the UK. The company’s $600 million funding round in 2021, led by SoftBank, marked the largest-ever private investment in the global medtech sector.
Mark Slack, CMR’s chief medical officer and co-founder, highlighted the importance of the approval: “Securing FDA marketing authorisation for Versius is a significant milestone for CMR and, most importantly, for hospitals and patients who will now have greater access to robotic-assisted surgery.”
Beyond the US, CMR Surgical is also seeking regulatory approval in other major healthcare markets, including Japan and China.
Although the company had previously considered an initial public offering (IPO), no formal plans have been announced. An IPO remains an option for the future as CMR continues its expansion into key international markets.
Founded in 2014, CMR Surgical has grown rapidly, advancing the accessibility and efficiency of robotic-assisted surgery globally. Its Versius system now stands as a key competitor in the burgeoning market for medical robotics, with the latest FDA approval set to enhance its presence on the global stage.